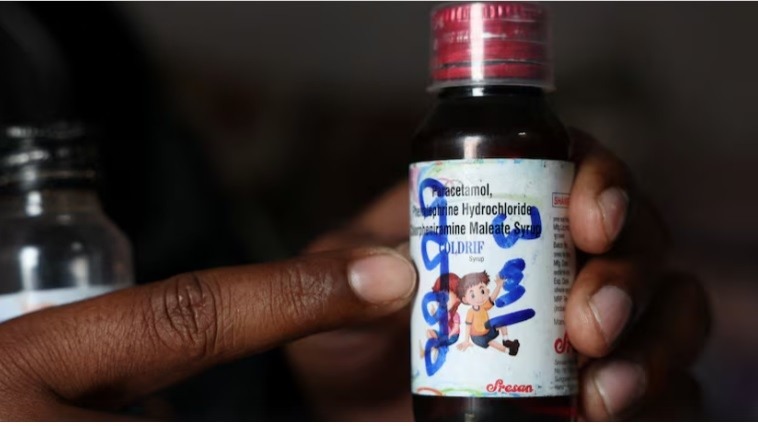
After several children died from contaminated cough syrup, the Indian government has taken a strict stance against pharmaceutical manufacturers. Authorities have ordered all drug factories across the country to modernize their production units to meet World Health Organization (WHO) standards by December — with no extensions allowed this time.
Government sources said the deadline for large pharmaceutical companies had already expired in June this year. For small and medium enterprises (MSMEs), the deadline was set for December 2024. However, after the discovery of the toxic chemical diethylene glycol (DEG) in the “Coldrif” cough syrup, the health ministry decided not to grant any further delays.
According to a Reuters report, the DEG level in the syrup was about 500 times higher than the permissible limit. The product was manufactured by Sresan Pharmaceuticals in Tamil Nadu. Several children in Madhya Pradesh died after consuming the syrup. The company’s production license has been revoked, and its founder has been arrested.
India’s Central Drugs Standard Control Organisation (CDSCO) has announced that in future, all raw materials and every batch of finished medicines must undergo mandatory testing. State-level drug authorities have also been instructed to intensify inspections of production facilities.
A senior government official stated:
“People are dying because of substandard production. That’s why no more time extensions will be provided.”
India currently has about 3,000 pharmaceutical companies and more than 10,000 production units. The government has warned that any company failing to upgrade its facilities on time will face an immediate shutdown.
Meanwhile, the World Health Organization (WHO) has expressed concern over the repeated incidents involving Indian cough syrups and has called for tighter oversight of exported pharmaceutical products.
— Saju / NAE